Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard
Adverse events should also be reported to Takeda UK Ltd. at: AE.GBR-IRL@takeda.com.
Prescribing information and adverse event reporting
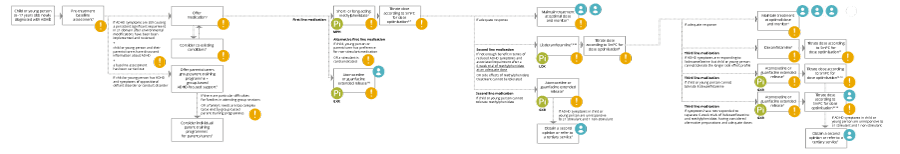
Please scroll through the treatment optimisation algorithm from left to right, following the appropriate treatment pathway for your patient aged 6–17 years newly diagnosed with ADHD.
At each step in the pathway, supporting information is available by clicking on the orange ‘Read more’ icon and illustrative expert clinical case studies are accessible by clicking on the blue ‘Case study’ icons at the end of individual treatment pathways. References are viewable at any time by clicking on the pink ‘References’ icon.
Welcome!
This toolkit is optimized for use on a Desktop.
Please use this toolkit from a desktop or laptop computer to have an improved experience.
Copyright 2025 Takeda Pharmaceutical Company Limited. All rights reserved.
Takeda and the Takeda Logo are registered trademarks of Takeda Pharmaceutical Company Limited.
C-APROM/GB/NS/1353 March 2025

Read More
- Before starting medication for attention deficit hyperactivity disorder (ADHD), people with ADHD should have a full assessment, which should include: 1
- A review to confirm they continue to meet the criteria for ADHD and need treatment
- A review of mental health and social circumstances, including:
- Presence of coexisting mental health and neurodevelopmental conditions
- Current educational or employment circumstances
- Risk assessment for substance misuse and drug diversion
- Care needs
- A review of physical health, including:
- A medical history, taking into account conditions that may be contraindications for specific medicines
- Current medication
- Height and weight (measured and recorded against the normal range for age, height and sex)
- Baseline pulse and blood pressure (measured with an appropriately sized cuff and compared with the normal range for age)
- A cardiovascular assessment
- An electrocardiogram (ECG) is not needed before starting stimulants, atomoxetine or guanfacine, unless the person has any of the features listed below, or a co-existing condition that is being treated with a medicine that may pose an increased cardiac risk
- Refer for a cardiology opinion before starting medication for ADHD if any of the following apply: 1
- History of congenital heart disease or previous cardiac surgery
- History of sudden death in a first-degree relative under 40 years suggesting a cardiac disease
- Shortness of breath on exertion compared with peers
- Fainting on exertion or in response to fright or noise
- Palpitations that are rapid, regular and start and stop suddenly (fleeting occasional bumps are usually ectopic and do not need investigation)
- Chest pain suggesting cardiac origin
- Signs of heart failure
- A murmur heard on cardiac examination
- Refer to a paediatric hypertension specialist before starting medication for ADHD if blood pressure is consistently above the 95th centile for age and height for children and young people 1
- Baseline ADHD symptoms and impairment should be measured and recorded using standard scales (e.g. Swanson, Nolan and Pelham Rating Scale Version IV [SNAP-IV] or ADHD Rating Scale Version 5 [ADHD-RS-5]) 14-16
- Ensure that children and young people with ADHD have a comprehensive, holistic shared treatment plan that addresses psychological, behavioural and occupational or educational needs. Take into account: 1
- The severity of ADHD symptoms and impairment, and how these affect or may affect everyday life (including sleep)
- Their goals
- Their resilience and protective factors
- The relative impact of other neurodevelopmental or mental health conditions
- Before starting any treatment for ADHD, discuss the following with the child or young person, and their family or carers as appropriate, encouraging them to give their own account of how they feel: 1
- The benefits and harms of non-pharmacological and pharmacological treatments
- The benefits of a healthy lifestyle, including exercise
- Their preferences and concerns
- How other mental health or neurodevelopmental conditions might affect treatment choices
- The importance of adherence to treatment and any factors that may affect this
- Record the person’s preferences and concerns in their treatment plan
Read More
- Environmental modifications are changes that are made to the physical environment in order to minimise the impact of a person’s attention deficit hyperactivity disorder (ADHD) on their day-to-day life 1
- Appropriate environmental modifications will be specific to the circumstances of each person with ADHD and should be determined from an assessment of their needs 1
- Examples may include: 1
- Changes to seating arrangements
- Changes to lighting and noise
- Reducing distractions
- Optimising education or work to have shorter periods of focus with movement breaks
- Reinforcing verbal requests with written instructions
-
The appropriate use of teaching assistants at school, for children
Read More
- All medication for attention deficit hyperactivity disorder (ADHD) should only be initiated by a healthcare professional with training and expertise in diagnosing and managing ADHD 1
- Healthcare professionals initiating medication for ADHD should: 1
- Be familiar with the pharmacokinetic profiles of all the short- and long-acting preparations available for ADHD
- Ensure that treatment is tailored effectively to the individual needs of the child or young person
-
Take account of variations in bioavailability or pharmacokinetic profiles of different preparations to avoid reduced effect or excessive adverse effects
Read More
- Offer the same medication choices to people with attention deficit hyperactivity disorder (ADHD) and anxiety disorder, tic disorder or autism spectrum disorder as other people with ADHD 1
- For children or young people with ADHD experiencing an acute psychotic or manic episode: 1
- Stop any medication for ADHD
- Consider restarting or starting new ADHD medication after the episode has resolved, taking into account the individual circumstances, risks and benefits of the ADHD medication
- If a person taking stimulants develops tics, think about whether: 1
- The tics are related to the stimulant (tics naturally wax and wane) and
- The impairment associated with the tics outweighs the benefits of the ADHD treatment
- If tics are stimulant related, reduce the stimulant dose, or consider changing to guanfacine extended release, atomoxetine, clonidine* or stopping medication 1
- If a person with ADHD develops new seizures or a worsening of existing seizures, review their medication and stop any medication that might be contributing to the seizures. After investigation, cautiously reintroduce ADHD medication if it is unlikely to be the cause of the seizures 1
*Clonidine is not licensed for the treatment of ADHD in the United Kingdom.
Read More
- Particular difficulties for families in attending group sessions, e.g. because of: 1
- Disability
- Needs related to diversity such as language differences
- Learning disability (intellectual disability)
- Parental ill-health
- Problems with transport
-
Or where other factors suggest poor prospects for therapeutic engagement
Read More
- Methylphenidate is indicated as part of a comprehensive treatment programme for attention deficit hyperactivity disorder (ADHD) in children aged 6 years of age and over when remedial measures alone prove insufficient 2-7
- A recent systematic review and network meta-analysis on the comparative efficacy and tolerability of medications for ADHD supported methylphenidate in children and young people as the preferred first choice medication for the short-term treatment of ADHD, taking into account both efficacy and safety 17
- The study authors also highlighted that new research should be funded urgently to assess the long-term effects of medications for ADHD
- When prescribing stimulants for ADHD, think about modified-release once-daily preparations for the following reasons: 1
- Convenience
- Improving adherence
- Reducing stigma (because there is no need to take medication at school or in the workplace)
- Reducing problems of storing and administering controlled drugs at school
- The risk of stimulant misuse and diversion with immediate-release preparations
- Their pharmacokinetic profiles
- Immediate-release preparations may be suitable if more flexible dosing regimens are needed, or during initial titration to determine correct dosing levels 1
- When prescribing stimulants for ADHD, be aware that effect size, duration of effect and adverse effects vary from person to person 1
- Very common (≥1/10) side effects reported with methylphenidate formulations are insomnia, nervousness and headache 2-7
- Think about using immediate- and modified-release preparations of stimulants to optimise effect (e.g. a modified-release preparation of methylphenidate in the morning and an immediate-release preparation of methylphenidate at another time of the day to extend the duration of effect) 1
- Be cautious about prescribing stimulants for ADHD if there is a risk of diversion for cognitive enhancement or appetite suppression 1
- Do not offer immediate-release stimulants or modified-release stimulants that can be easily injected or insufflated if there is a risk of stimulant misuse or diversion 1
- Please see the individual Summary of Product Characteristics for detailed information on starting treatment with methylphenidate. 2-7
Read More
- Before starting any treatment for attention deficit hyperactivity disorder (ADHD), discuss the following with the person and their family or carers as appropriate, encouraging children and young people to give their own account of how they feel: 1
-
Their preferences and concerns (it is important to understand that a person’s decision to start, change or stop treatment
may be influenced by media coverage, teachers, family members, friends and differing opinion on the validity of a diagnosis of ADHD) - Record the person’s preferences and concerns in their treatment plan
- Contraindications to stimulants include:
Methylphenidate 2-7
- Hypersensitivity to methylphenidate or to any of the excipients
- Glaucoma
- Phaeochromocytoma
- During treatment with non-selective, irreversible monoamine oxidase inhibitors (MAOI), or within a minimum of 14 days of discontinuing those drugs, due to the risk of hypertensive crisis
- Hyperthyroidism or thyrotoxicosis
- Diagnosis or history of severe depression, anorexia nervosa/anorexic disorders, suicidal tendencies, psychotic symptoms, severe mood disorders, mania, schizophrenia, psychopathic/borderline personality disorder
- Diagnosis or history of severe and episodic (Type I) Bipolar (affective) Disorder (that is not well-controlled)
- Pre-existing cardiovascular disorders including severe hypertension, heart failure, arterial occlusive disease, angina, haemodynamically significant congenital heart disease, cardiomyopathies, myocardial infarction, potentially life-threatening arrhythmias and channelopathies (disorders caused by the dysfunction of ion channels)
- Pre-existing cerebrovascular disorders cerebral aneurysm, vascular abnormalities including vasculitis or stroke
Lisdexamfetamine 8
- Hypersensitivity to sympathomimetic amines or any of the excipients
- Concomitant use of monoamine oxidase inhibitors (MAOI) or within 14 days after MAOI treatment (hypertensive crisis may result)
- Hyperthyroidism or thyrotoxicosis
- Agitated states
- Symptomatic cardiovascular disease
- Advanced arteriosclerosis
- Moderate to severe hypertension
- Glaucoma
Dexamfetamine 11
- Known hypersensitivity to the active substance or any of the excipients
- Known hypersensitivity to sympathomimetic amines
- Glaucoma
- Phaeochromocytoma
- Symptomatic cardiovascular disease, structural cardiac abnormalities and/or moderate or severe hypertension, heart failure, arterial occlusive disease, angina, haemodynamically significant congenital heart disease, cardiomyopathies, myocardial infarction, potentially life-threatening arrhythmias and channelopathies (disorders caused by the dysfunction of ion channels)
- Advanced arteriosclerosis
- Concomitant use of MAOI or within 14 days of MAOI treatment
- Hyperthyroidism or thyrotoxicosis
- Severe depression, anorexia nervosa/anorexic disorders, suicidal ideation, hyperexcitability, psychotic symptoms, severe and episodic (Type I) Bipolar (affective) Disorder (that is not well-controlled), schizophrenia, psychopathic/borderline personality disorder
- Gilles de la Tourette syndrome or similar dystonias
- Cerebrovascular disorders (cerebral aneurysm, vascular abnormalities including vasculitis or stroke)
- Porphyria
- History of drug abuse or alcohol abuse
Please see the individual Summary of Product Characteristics for further information. 2-8,11
Read More
- Atomoxetine and guanfacine extended release are non-stimulant treatment options for children and young people with attention deficit hyperactivity disorder (ADHD) with different hypothesised modes of action, times to response, durations of action and adverse event profiles 12-13,18-19
Atomoxetine
- Atomoxetine is indicated for the treatment of ADHD in children of 6 years and older as part of a comprehensive treatment programme 12
- Very common (≥1/10) side effects reported with atomoxetine are appetite decreased, headache, somnolence, abdominal pain, vomiting, nausea, blood pressure increased and heart rate increased 12
Guanfacine extended release
- Guanfacine extended release is indicated for the treatment of ADHD in children and young people 6–17 years old for whom stimulants are not suitable, not tolerated or have been shown to be ineffective 13
- Very common (≥1/10) side effects reported with guanfacine extended release are somnolence, headache, abdominal pain and fatigue 13
-
Please see the individual Summary of Product Characteristics for detailed information on starting treatment with atomoxetine or guanfacine extended release.
12,13
Read More
- Atomoxetine and guanfacine extended release are non-stimulant treatment options for children and young people with attention deficit hyperactivity disorder (ADHD) with different hypothesised modes of action, times to response, durations of action and adverse event profiles 12-13,18-19
Atomoxetine
- Atomoxetine is indicated for the treatment of ADHD in children of 6 years and older as part of a comprehensive treatment programme 12
- Very common (≥1/10) side effects reported with atomoxetine are appetite decreased, headache, somnolence, abdominal pain, vomiting, nausea, blood pressure increased and heart rate increased 12
Guanfacine extended release
- Guanfacine extended release is indicated for the treatment of ADHD in children and young people 6–17 years old for whom stimulants are not suitable, not tolerated or have been shown to be ineffective 13
- Very common (≥1/10) side effects reported with guanfacine extended release are somnolence, headache, abdominal pain and fatigue 13
Please see the individual Summary of Product Characteristics for detailed information on starting treatment with atomoxetine or guanfacine extended release.
12,13
Read More
- Atomoxetine and guanfacine extended release are non-stimulant treatment options for children and young people with attention deficit hyperactivity disorder (ADHD) with different hypothesised modes of action, times to response, durations of action and adverse event profiles 12-13,18-19
Atomoxetine
- Atomoxetine is indicated for the treatment of ADHD in children of 6 years and older as part of a comprehensive treatment programme 12
- Very common (≥1/10) side effects reported with atomoxetine are appetite decreased, headache, somnolence, abdominal pain, vomiting, nausea, blood pressure increased and heart rate increased 12
Guanfacine extended release
- Guanfacine extended release is indicated for the treatment of ADHD in children and young people 6–17 years old for whom stimulants are not suitable, not tolerated or have been shown to be ineffective 13
- Very common (≥1/10) side effects reported with guanfacine extended release are somnolence, headache, abdominal pain and fatigue 13
Please see the individual Summary of Product Characteristics for detailed information on starting treatment with atomoxetine or guanfacine extended release.
12,13
Read More
- Atomoxetine and guanfacine extended release are non-stimulant treatment options for children and young people with attention deficit hyperactivity disorder (ADHD) with different hypothesised modes of action, times to response, durations of action and adverse event profiles 12-13,18-19
Atomoxetine
- Atomoxetine is indicated for the treatment of ADHD in children of 6 years and older as part of a comprehensive treatment programme 12
- Very common (≥1/10) side effects reported with atomoxetine are appetite decreased, headache, somnolence, abdominal pain, vomiting, nausea, blood pressure increased and heart rate increased 12
Guanfacine extended release
- Guanfacine extended release is indicated for the treatment of ADHD in children and young people 6–17 years old for whom stimulants are not suitable, not tolerated or have been shown to be ineffective 13
- Very common (≥1/10) side effects reported with guanfacine extended release are somnolence, headache, abdominal pain and fatigue 13
Please see the individual Summary of Product Characteristics for detailed information on starting treatment with atomoxetine or guanfacine extended release.
12,13
Read More
- Several different short- and long-acting methylphenidate preparations are licensed and available for the treatment of attention deficit hyperactivity disorder (ADHD) in children and young people in the United Kingdom as part of a comprehensive treatment programme when remedial measures alone prove insufficient 2-7
- Each formulation has its own approved titration dosing regimen
- Please see the individual Summary of Product Characteristics for detailed information on posology
- During the titration phase, ADHD symptoms, impairment and adverse effects should be recorded at baseline and at each dose change on standard scales by parents and teachers, and progress reviewed regularly with a specialist 1
- Titrate the dose against symptoms and adverse effects in line with the licensed titration dosing regimen until dose optimisation is achieved, that is, reduced symptoms, positive behaviour change, improvements in education, employment and relationships, with tolerable adverse effects 1
- Ensure that dose titration is slower and monitoring more frequent if any of the following are present in people with ADHD: 1
- Neurodevelopmental disorders
- Mental health conditions
-
Physical health conditions
Read More
- Monitor effectiveness of medication for attention deficit hyperactivity disorder (ADHD) and adverse effects, and document in the person’s notes 1
- Encourage people taking medication for ADHD to monitor and record their adverse effects 1
- Consider using standard symptom and adverse effect rating scales for clinical assessment and throughout the course of treatment for people with ADHD1 (e.g. Swanson, Nolan and Pelham Rating Scale Version IV [SNAP-IV] or ADHD Rating Scale Version 5 [ADHD-RS-5]) 14-16
- Ensure that children and young people receiving treatment for ADHD have review and follow-up according to the severity of their condition, regardless of whether or not they are taking medication 1
- Height and weight – For people taking medication for ADHD: 1
- Measure height every 6 months in children and young people
- Measure weight every 3 months in children 10 years and under
- Measure weight at 3 and 6 months after starting treatment in children over 10 years and young people, and every 6 months thereafter, or more often if concerns arise
- Plot height and weight of children and young people on a growth chart and ensure review by the healthcare professional responsible for treatment
- If weight loss is a clinical concern, consider the following strategies: 1
- Taking medication either with or after food, rather than before meals
- Taking additional meals or snacks early in the morning or late in the evening when stimulant effects have worn off
- Obtaining dietary advice
- Consuming high-calorie foods of good nutritional value
- Taking a planned break from treatment
- Changing medication
- If a child or young person’s height over time is significantly affected by medication (that is, they have not met the height expected for their age), consider a planned break in treatment over school holidays to allow ‘catch-up’ growth 1
-
For additional maintenance and monitoring advice related to cardiovascular health, tics, sexual dysfunction, seizures, sleep, worsening behaviour or stimulant diversion, see the National Institute for Health and Care Excellence (NICE) ADHD diagnosis and management guideline
1
Read More
- Monitor effectiveness of medication for attention deficit hyperactivity disorder (ADHD) and adverse effects, and document in the person’s notes 1
- Encourage people taking medication for ADHD to monitor and record their adverse effects 1
- Consider using standard symptom and adverse effect rating scales for clinical assessment and throughout the course of treatment for people with ADHD1 (e.g. Swanson, Nolan and Pelham Rating Scale Version IV [SNAP-IV] or ADHD Rating Scale Version 5 [ADHD-RS-5]) 14-16
- Ensure that children and young people receiving treatment for ADHD have review and follow-up according to the severity of their condition, regardless of whether or not they are taking medication 1
- Height and weight – For people taking medication for ADHD: 1
- Measure height every 6 months in children and young people
- Measure weight every 3 months in children 10 years and under
- Measure weight at 3 and 6 months after starting treatment in children over 10 years and young people, and every 6 months thereafter, or more often if concerns arise
- Plot height and weight of children and young people on a growth chart and ensure review by the healthcare professional responsible for treatment
- If weight loss is a clinical concern, consider the following strategies: 1
- Taking medication either with or after food, rather than before meals
- Taking additional meals or snacks early in the morning or late in the evening when stimulant effects have worn off
- Obtaining dietary advice
- Consuming high-calorie foods of good nutritional value
- Taking a planned break from treatment
- Changing medication
- If a child or young person’s height over time is significantly affected by medication (that is, they have not met the height expected for their age), consider a planned break in treatment over school holidays to allow ‘catch-up’ growth 1
-
For additional maintenance and monitoring advice related to cardiovascular health, tics, sexual dysfunction, seizures, sleep, worsening behaviour or stimulant diversion, see the National Institute for Health and Care Excellence (NICE) ADHD diagnosis and management guideline
1
Read More
- Lisdexamfetamine is indicated as part of a comprehensive treatment programme for attention deficit hyperactivity disorder (ADHD) in children aged 6 years and over when response to previous methylphenidate treatment is considered clinically inadequate 8
- A recent systematic review and network meta-analysis on the comparative efficacy and tolerability of medications for ADHD found that amfetamines were the most efficacious compounds in children and young people; however, amfetamines were less well tolerated than placebo in this age group 17
- The study authors also highlighted that new research should be funded urgently to assess the long-term effects of medications for ADHD
- When prescribing stimulants for ADHD, be aware that effect size, duration of effect and adverse effects vary from person to person 1
- Very common (≥1/10) side effects reported with lisdexamfetamine in children and adolescents are decreased appetite, insomnia, headache, upper abdominal pain and weight decreased 8
- Although there is considerable overlap in the adverse event profiles of methylphenidate and lisdexamfetamine,2-8 studies suggest that the frequency and severity of adverse events may differ between treatments.9,10 Individual patient response profiles are noncongruent and non-response or intolerable side effects with one stimulant does not preclude a good response to the other 9,10
- Be cautious about prescribing stimulants for ADHD if there is a risk of diversion for cognitive enhancement or appetite suppression 1
-
Please see the individual Summary of Product Characteristics for detailed information on starting treatment with lisdexamfetamine.
8
Read More
- The dosage of lisdexamfetamine should be individualised according to the therapeutic needs and response of the patient. Careful dose titration is necessary at the start of treatment 8
- The starting dose is 30 mg taken once daily in the morning. When in the judgment of the clinician a lower initial dose is appropriate, patients may begin treatment with 20 mg once daily in the morning 8
- The dose may be increased by 10 or 20 mg increments, at approximately weekly intervals. Lisdexamfetamine should be administered orally at the lowest effective dosage 8
- The maximum recommended dose is 70 mg/day; higher doses have not been studied 8
- Treatment must be stopped if the symptoms do not improve after appropriate dosage adjustment over a one-month period. If paradoxical aggravation of symptoms or other intolerable adverse events occur, the dosage should be reduced or discontinued 8
Please see the individual Summary of Product Characteristics for detailed information on posology. 8
Read More
- Dexamfetamine is indicated as part of a comprehensive treatment programme for attention deficit hyperactivity disorder (ADHD) in children and young people aged 6 to 17 years old when response to previous methylphenidate treatment is considered clinically inadequate 11
- A recent systematic review and network meta-analysis on the comparative efficacy and tolerability of medications for ADHD found that amfetamines were the most efficacious compounds in children and young people; however, amfetamines were less well tolerated than placebo in this age group 17
- The study authors also highlighted that new research should be funded urgently to assess the long-term effects of medications for ADHD
- When prescribing stimulants for ADHD, be aware that effect size, duration of effect and adverse effects vary from person to person 1
- Very common (≥1/10) side effects reported with dexamfetamine are decreased appetite, reduced weight gain and weight loss during prolonged use in children, insomnia and nervousness 11
- Be cautious about prescribing stimulants for ADHD if there is a risk of diversion for cognitive enhancement or appetite suppression 1
Please see the individual Summary of Product Characteristics for detailed information on starting treatment with dexamfetamine.
11
Read More
- Careful dose titration is necessary at the start of treatment with dexamfetamine. Dose titration should be started at the lowest possible dose 11
- The recommended starting daily dose is 5 mg once or twice daily (e.g. at breakfast and lunch), increasing if necessary by weekly increments of 5 mg in the daily dose according to tolerability and degree of efficacy observed 11
- In the treatment of attention deficit hyperactivity disorder (ADHD), the times at which the doses of dexamfetamine are administered should be selected to provide the best effect when it is most needed to combat school and social behavioural difficulties. Normally the first increasing dose is given in the morning. Dexamfetamine should not be taken too late after lunch time to avoid disturbances of sleep 11
- The regimen that achieves satisfactory symptom control with the lowest total daily dose should be employed 11
- The maximum daily dose in children and young people is usually 20 mg, although doses of 40 mg may in rare cases be necessary for optimum titration. The decision to give dexamfetamine once or twice daily should be based on the course of symptoms at different times of the day 11
Please see the individual Summary of Product Characteristics for detailed information on posology.
11
Read More
Atomoxetine12
- Atomoxetine can be administered as a single daily dose in the morning. Patients who do not achieve a satisfactory clinical response (tolerability [e.g. nausea or somnolence] or efficacy) when taking atomoxetine as a single daily dose might benefit from taking it as twice daily evenly divided doses in the morning and late afternoon or early evening
- Dosing of paediatric population up to 70 kg body weight:
- Atomoxetine should be initiated at a total daily dose of approximately 0.5mg/kg. The initial dose should be maintained for a minimum of 7 days prior to upward dose titration according to clinical response and tolerability. The recommended maintenance dose is approximately 1.2mg/kg/day (depending on the patient’s weight and available dosage strengths of atomoxetine). No additional benefit has been demonstrated for doses higher than 1.2mg/kg/day. The safety of single doses over 1.8mg/kg/day and total daily doses above 1.8 mg/kg have not been systematically evaluated. In some cases it might be appropriate to continue treatment into adulthood
- Dosing of paediatric population over 70 kg body weight:
- Atomoxetine should be initiated at a total daily dose of 40 mg. The initial dose should be maintained for a minimum of 7 days prior to upward dose titration according to clinical response and tolerability. The recommended maintenance dose is 80 mg. No additional benefit has been demonstrated for doses higher than 80 mg. The maximum recommended total daily dose is 100 mg. The safety of single doses over 120 mg and total daily doses above 150 mg have not been systematically evaluated
Guanfacine extended release 13
- Careful dose titration and monitoring is necessary at the start of treatment with guanfacine extended release since clinical improvement and risks for several clinically significant adverse reactions (syncope, hypotension, bradycardia, somnolence and sedation) are dose- and exposure-related. Patients should be advised that somnolence and sedation can occur, particularly early in treatment or with dose increases. If somnolence and sedation are judged to be clinically concerning or persistent, a dose decrease or discontinuation should be considered
- For all patients, the recommended starting dose is 1 mg of guanfacine, taken orally once a day
- The dose may be adjusted in increments of not more than 1 mg per week. Dose should be individualised according to the patient’s response and tolerability
-
Depending on the patient’s response and tolerability for guanfacine extended release the recommended maintenance dose range is 0.05–0.12 mg/kg/day. The recommended dose titration for children and young people is provided below (see tables 1 and 2). Dose adjustments (increase or decrease) to a maximum tolerated dose within the recommended optimal weight-adjusted dose range based upon clinical judgement of response and tolerability may occur at any weekly interval after the initial dose
Table 1
| Dose titration schedule for children (aged 6–12 years) | ||||
|---|---|---|---|---|
| Weight group | Week 1 | Week 2 | Week 3 | Week 4 |
| 25 kg and up Max dose = 4 mg | 1 mg | 2 mg | 3 mg | 4 mg |
Table 2
| Dose titration schedule for young people (aged 13–17 years) | |||||||
|---|---|---|---|---|---|---|---|
| Weight group | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 |
| 34–41.4 kg Max dose = 4 mg | 1 mg | 2 mg | 3 mg | 4 mg | |||
| 41.5–49.4 kg Max dose = 5 mg | 1 mg | 2 mg | 3 mg | 4 mg | 5 mg | ||
| 49.5–58.4 kg Max dose = 6 mg | 1 mg | 2 mg | 3 mg | 4 mg | 5 mg | 6 mg | |
| 58.5 kg and above Max dose = 7 mg | 1 mg | 2 mg | 3 mg | 4 mg | 5 mg | 6 mg | 7 mg |
- Young persons must weigh at least 34 kg.
2. Young persons weighing 58.5 kg and above may be titrated to a 7 mg/day dose after the subject has completed a minimum of 1 week of therapy on a 6 mg/day dose and the physician has performed a thorough review of the subject’s tolerability and efficacy.
Please see the individual Summary of Product Characteristics for detailed information on posology.
12,13
More info
Give your visitors more information about a menu item, new product or team member, all without taking up room on the page.
Describe special ingredients in a new dish, write about the virtues of a unique product, or provide someone’s personal bio.
Welcome!
This toolkit is optimized for use on a Desktop.
Please use this toolkit from a desktop or laptop computer to have an improved experience.

Welcome!
This toolkit is optimized for use on a Desktop.
Please use this toolkit from a desktop or laptop computer to have an improved experience.
References
- National Institute for Health and Care Excellence (NICE). Attention deficit hyperactivity disorder: diagnosis and management. NICE guideline 87. 2018 [Updated April 2023]. Available at: https://www.nice.org.uk/guidance/ng87/chapter/Update-information [Accessed March 2025]
- Flynn Pharma Ltd. Medikinet UK SmPC. Last updated 09 January 2023. [Accessed March 2025]
- Zentiva Pharma Ltd. Affenid XL SmPC. Last updated 05 April 2023. [Accessed March 2025]
- Flynn Pharma Ltd. Medikinet XL UK SmPC. Last updated 09 January 2023. [Accessed March 2025]
- Advanz Pharma Ltd. Addepta XL SmPc. Last updated 09 November 2021. [Accessed March 2025]
- Janssen Cilag Ltd. Concerta XL UK SmPC. Last updated 11 November 2022. [Accessed March 2025]
- Takeda UK Ltd. Equasym XL SmPC. Last updated 11 November 2022. [Accessed March 2025]
- Takeda UK Ltd. Elvanse UK SmPC. Last updated 14 November 2022. [Accessed March 2025]
- Arnold LE. Methylphenidate vs. amphetamine: comparative review. J Atten Disord 2000; 3(4): 200–211.
- Hodgkins P, Shaw M, Coghill D, Hechtman L. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry 2012; 21(9): 477–492.
- Flynn Pharma Ltd. Amfexa UK SmPC. Last updated 13 January 2023. [Accessed March 2025]
- Eli Lilly & Company Ltd. Strattera UK SmPC. Last updated 08 Feb 2021. [Accessed March 2025]
- Takeda UK Ltd. Intuniv UK SmPC. Last updated 06 February 2023. [Accessed March 2025]
- Case study clinical opinion.
- SNAP-IV Teacher and Parent 18-Item Rating Scale. James M. Swanson, Ph.D., University of California, Irvine, CA 92715. Available at: http://www.shared-care.ca/files/Scoring_for_SNAP_IV_Guide_18-item.pdf [Accessed March 2025]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale – 5 for Children and Adolescents: Checklists, norms, and clinical interpretation. New York: Guildford. 2016.
- Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review of network meta-analysis. Lancet Psychiatry 2018; 5(9): 727–738.
- Hervas A, Huss M, Johnson M, et al. Efficacy and safety of extended-release guanfacine hydrochloride in children and adolescents with attention-deficit/hyperactivity disorder: A randomized, controlled, Phase III trial. Eur Neuropsychopharmacol 2014; 24(12): 1861–1872.
-
Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 2008; 121(1): e73–e84.